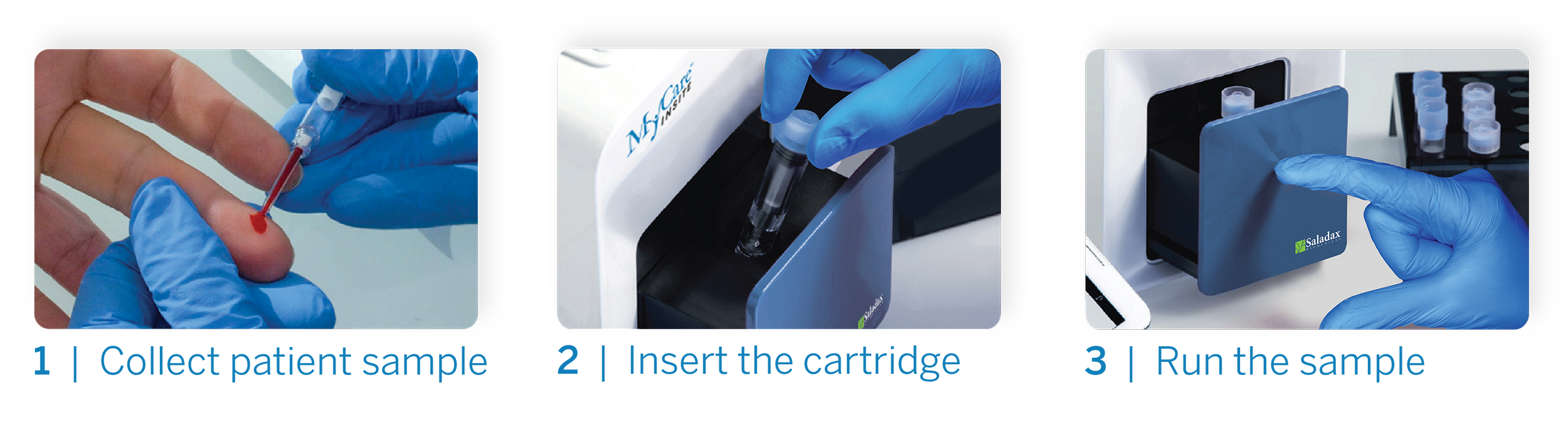
Acceptability of point of care testing for antipsychotic medication levels in schizophrenia
Atkins M, Taylor D, Harland R, Brewer A, Williams S, Chesney E, McGuire P. Psych Research Com. 2022 https://doi.org/10.1016/j.psycom.2022.100070
ABSTRACT
We surveyed 106 patients with schizophrenia who were being treated with either oral clozapine or oral aripiprazole. For each patient, the plasma level of the medication was measured using i) a venous blood sample and a conventional lab-based assay and ii) a novel point of care assay that used a capillary blood sample taken with a finger prick. Immediately after providing the two samples, participants completed a brief questionnaire. We also surveyed 10 members of staff who were directly involved in the care of these patients. 98% of patients found the capillary point-of-care approach acceptable, and 85% preferred it to the conventional venous blood procedure. 78% of patients said it was useful to have access to the result at the point of care (as opposed to at a later date), and 90% felt that POC testing improved clinical care. 83% said that the POC test made them feel more involved in their treatment. 100% of staff said their experience with the POC test was good, that it was easier than venous collection, and that it was very useful to receive the medication level while the patient was still in the clinic.