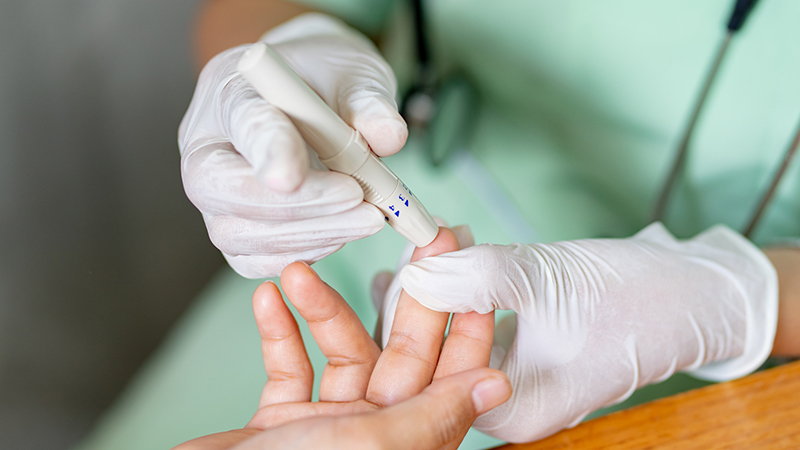
Bethlehem, PA – September 05, 2019 –Saladax Biomedical today announced that its MyCare Insite Clozapine Test is now CE marked for use on the MyCare Insite. The MyCare Insite Clozapine Test is the first and only rapid point-of-care test CE marked to aid mental health professionals in the treatment and monitoring of patients with schizophrenia.
The MyCare Insite Clozapine Test delivers accurate and precise clozapine blood levels comparable to laboratory results. Psychiatrists need immediate information to make informed decisions during a patient visit. The MyCare Insite Clozapine Test addresses this unmet medical need by providing answers in only six minutes. Clinicians, in consultation with their patients, can use these results to make real-time adjustments to better manage their patients.
“Mental health professionals have very few tools to aid in patient treatment; as a result, patients are suffering. The MyCare Insite Clozapine Test provides clinicians a first of its kind device. The test is evidence of how Saladax is once again setting new standards for innovation in the treatment and monitoring of patients,” said Dr. Salvatore Salamone, CEO and Founder of Saladax Biomedical. “We are changing psychiatry with a single drop of blood.”
ABOUT PSYCHOSIS
Psychosis is a condition that affects the way your brain processes information. It causes you to lose touch with reality. Psychotic disorders, like schizophrenia, involve psychosis that usually affects you for the first time in the late teen years or early adulthood. Young people are especially at risk, but doctors don’t know why.
ABOUT THE MyCARE INSITE CLOZAPINE TEST
The MyCare Insite Clozapine Test is intended for the in vitro quantitative measurement of clozapine in human capillary finger blood using the MyCare Insite.
View the original press release at https://www.businesswire.com/news/home/20190905005085/en/Saladax-Develops-First-Ever-Rapid-Test-Antipsychotic-Drug