The MyCare Oncology Busulfan Assay Kit provides rapid results so that busulfan exposure can be reported and acted upon before the next dose

Using body weight alone to determine busulfan dosing is insufficient. With the MyCare Busulfan Assay, receive busulfan drug levels during treatment to greatly increase confidence that optimum exposure has been achieved.
Only 60 – 70% of patients achieve target busulfan exposure when dosed by weight1
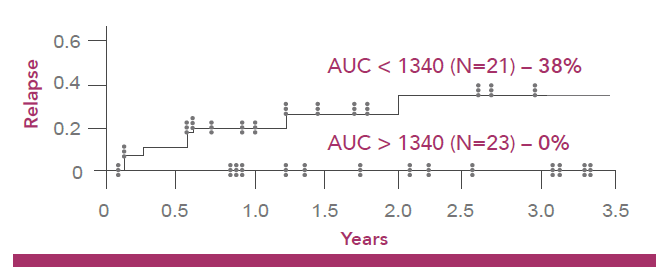
Risk to the patient not achieving optimal busulfan exposure (900 – 1340 µM min):2
- Risks of high exposure:
- Mucositis
- Graft vs. host disease
- Veno-occlusive disease
- Transplantation-related mortality
- Risks of low exposure:
- Graft rejection
- Disease relapse
Improved Outcomes with Busulfan Monitoring
Lower incidence of disease relapse 3 years post transplantation3
Lower incidence of Veno-occlusive disease (VOD):4
-
- With real-time dose adjustment: 0 – 5%
- Without real-time dose adjustment: 24 – 75%
About Busulfan Testing
- Busulfan is used before hematopoietic stem cell transplantation (HSCT)5
- HSCT is the standard treatment in various malignant and non-malignant diseases including leukemia (acute and chronic myeloid, acute lymphocytic), non-Hodgkin’s lymphoma, Hodgkin’s disease, metabolic diseases, immunodeficiencies, and hemoglobinopathy
- Currently, busulfan dosing is determined by a patient’s body weight
- Busulfan exposure is calculated as area under the time versus concentration curve (AUC)
- Optimal exposure is essential for successful hematopoietic stem cell transplantation outcomes
References
1. Palmer et al. 2016. Biol Blood Marrow Transplant; 22: 1915-1925.
2. Busulfex Package Insert
3. Slattery et al. 1997. Blood; 89: 3055-3060.
4. Grochow. 1993. Semin Oncol; 20: 18-25.
5. Bartelink et al. 2016. Lancet Haematol; 3: e526-536.
Busulfan Assay Performance
Precision Data
| Sample Type | Assigned Value (ng/mL) | N | Mean (ng/mL) | Repeatability %CV | Within-Laboratory %CV | |
|---|---|---|---|---|---|---|
| Controls | Low | 225 | 80 | 250 | 4.6 | 6.1 |
| Medium | 450 | 80 | 461 | 3.1 | 3.9 | |
| High | 900 | 80 | 910 | 1.8 | 2.8 | |
| Plasma | Spike 1 | 325 | 80 | 328 | 4.7 | 5.7 |
| Spike 2 | 600 | 80 | 615 | 4 | 4.8 | |
| Spike 3 | 1100 | 80 | 1124 | 2.1 | 2.9 | |
| Spike 4 | 1500 | 80 | 1531 | 2.6 | 3.1 | |
Method Comparison
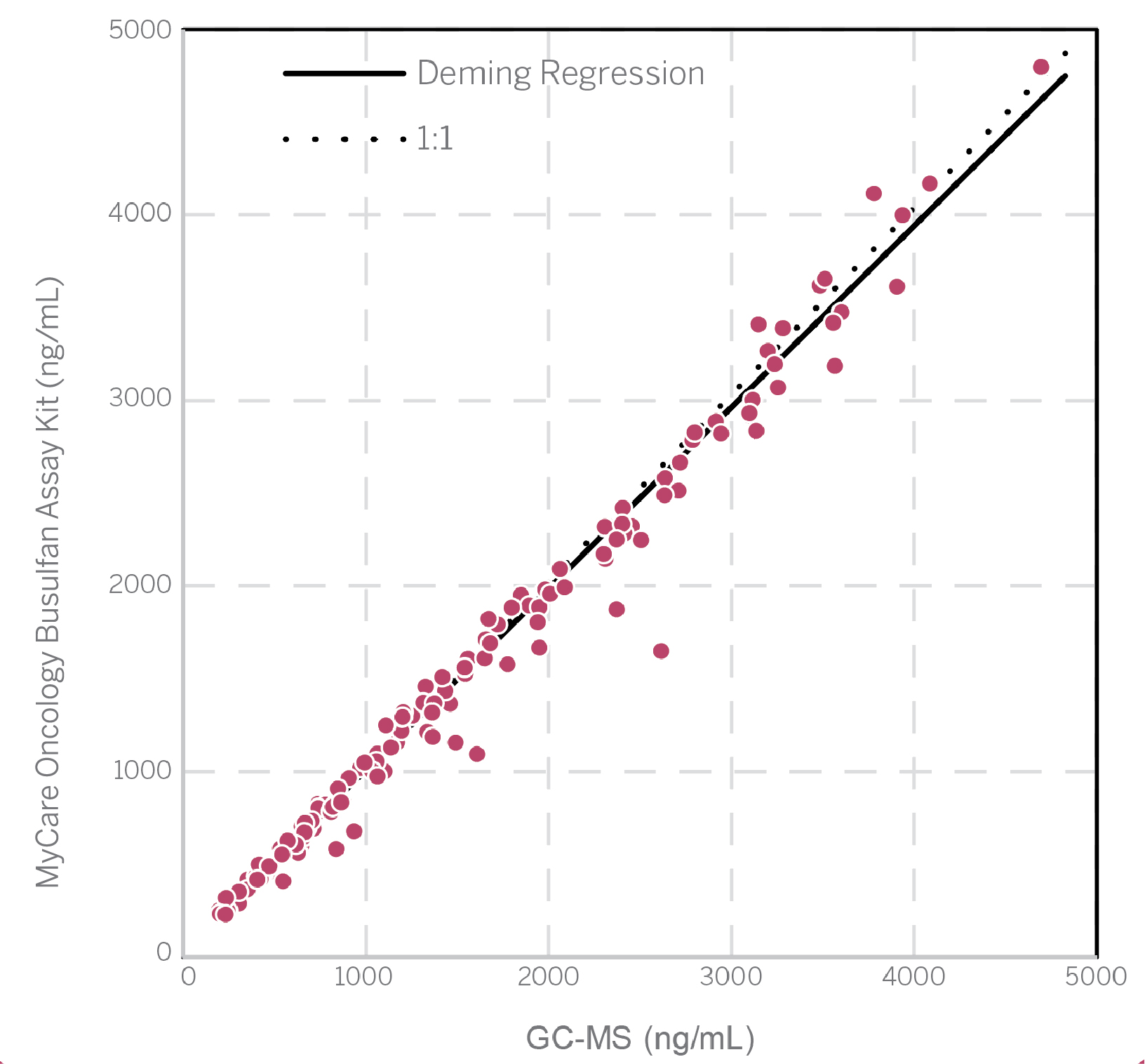
Slope = 0.97, Y-Intercept = 18
Correlation Coefficient (r) = 0.9917, N = 208
Talk to Your Doctor
Ask about Busulfan by Saladax
Reduce adverse effects & gain a longer progression free survival.
Request Information
You can also contact our team by completing and submitting our contact form. We will get back to you shortly.


